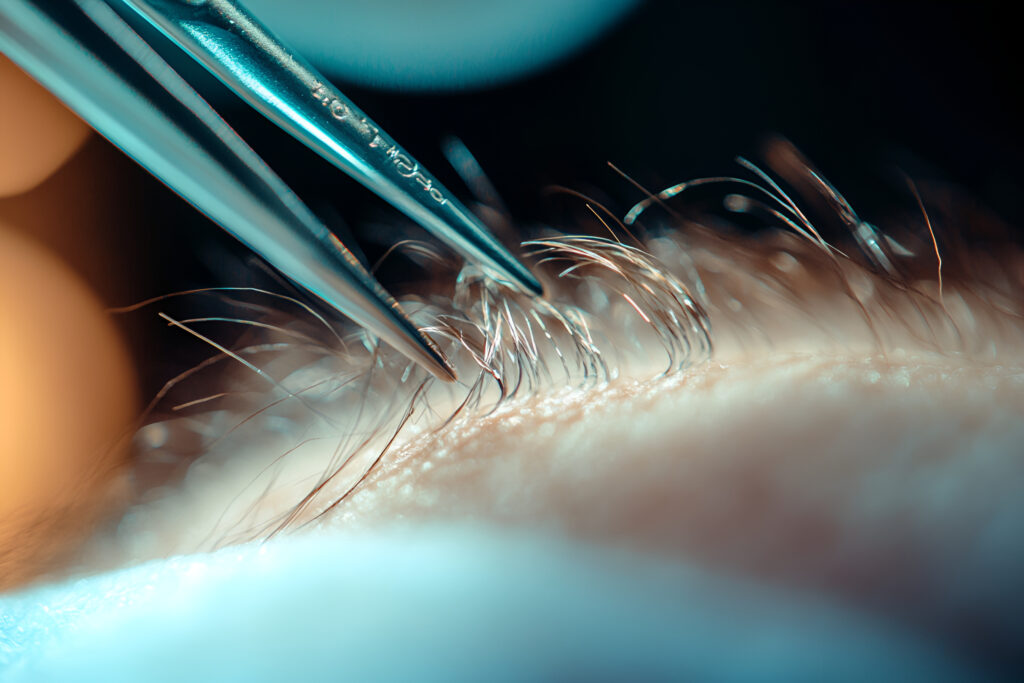
A first-in-human trial suggests ET-02 could be a promising novel topical treatment for androgenic alopecia. Participants using the 5% ET-02 solution saw a six-fold increase in non-vellus hair count within five weeks.
Mechanism of Action
ET-02 (Eirion Therapeutics Inc.) is a topical small molecule designed to target and restore hair follicle stem cells, which are believed to play a key role in regulating hair growth but may become defective or inactive in androgenic alopecia (age-related hair loss).1,2 By addressing this stem cell dysfunction, it is hoped ET-02 will stimulate hair regrowth and potentially do so more effectively than current treatments, which do not directly target the underlying cause of this type of hair loss.2
In addition, unlike some available treatments, as ET-02 does not target hormonal pathways, it is therefore not expected to cause side effects such as sexual dysfunction, which are commonly associated with androgen-inhibiting therapies like finasteride.3
Study Design2
This phase 1, double-blind, placebo-controlled, dose-ranging trial enrolled 24 male participants across three investigational sites in the USA. Participants were randomly assigned 1:1:1 to receive either placebo (vehicle-only), 1.25% ET-02 solution, or 5% ET-02 solution, applied once daily for four weeks. A final assessment was conducted one week post-treatment, and these initial results were shared in a press release from the manufacturers and discussed in a recent interview with Jon Edelson, CEO of Eirion.
Key Findings2,4
- Safety & Tolerability: ET-02 was found to be well tolerated, with no local skin reactions (e.g., erythema, itching, burning, or scaling), no ocular irritation, and no significant abnormalities in bloodwork or electrocardiograms.
- Dose response: A dose-response relationship was observed. Minimal response was reported in the placebo and 1.25% ET-02 groups so both groups were combined in the analysis.
- Hair Growth: The 5% ET-02 group experienced about a six-fold increase in non-vellus (normal) hair count compared to placebo.
- Hair Thickness: The 5% ET-02 group showed an approximately 10% increase in hair width over the placebo group.
- Speed of Results: Hair growth was observed in five weeks.
Comparative Efficacy
The results of this trial are consistent with findings from ET-02’s earlier preclinical study that tested 5% ET-02 on 60 human scalp tissue grafts from men with androgenic alopecia.2 In that study, ET-02 also demonstrated greater effectiveness than the control group. Notably, Eirion Therapeutics reported that by the fourth month of treatment, the net rate of hair growth with ET-02 was four times higher than that observed with minoxidil in a separate preclinical study (n=103) using the same experimental graft model.
Commenting on the results so far, Dr Jerry Shapiro, an expert in hair loss and Professor of Dermatology at New York University Grossman School of Medicine, stated:
“Achieving this amount of hair growth in just 5 weeks in a clinical trial is unprecedented. ET-02 represents a potentially substantial advancement over minoxidil and other commercially available pharmaceuticals for patients struggling with hair loss—not only from an efficacy standpoint but also in terms of safety and ease of use.”
Conclusion & Next Steps
These early data suggest that ET-02 could represent a promising new approach to hair loss treatment—one that focuses on restoring follicular stem cell function rather than merely stimulating hair growth. By addressing this underlying mechanism, ET-02 may not only promote hair regrowth but also help prevent androgenic alopecia.
To further investigate its potential, a larger phase 2 clinical trial (n≈150) is planned for later this year, with a six-month treatment period to assess its long-term effects.
References
- Egger A, Tomić-Čaníc M, Tosti A. Advances in Stem Cell-Based Therapy for Hair Loss. CellR4 Repair Replace Regen Reprogram. 2020;8:e2894. Epub 2020 Sep 2.
- Eirion Therapeutics. Eirion Therapeutics Announces Potential Breakthrough Treatment for Hair Loss Based on First-in-Man Clinical Trial Results [Press release]. Available at: https://www.eirionthera.com/single-post/eirion-therapeutics-announces-potential-breakthrough-treatment-for-hair-loss-based-on-first-in-man-c. (accessed 30 Jan 2025)
- Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J. 2012 Jan;3(1):62-5. doi: 10.4103/2229-5178.93496.
- Dermatology Times. The Unprecedented Phase 1 Results of ET-02 for the Treatment of Androgenic Alopecia [News article]. Available at: The-unprecedented-phase-1-results-of-et-02-for-the-treatment-of-androgenic-alopecia (accessed 30 Jan 2025)
Click here for further content in the field of alopecia and aesthetic dermatology.
***
SIGN UP to TouchDERMATOLOGY!
Join our global community today for access to thousands of peer-reviewed articles, expert insights, and learn-on-the-go education across 150+ specialties, plus concise email updates and newsletters so you never miss out.
REGISTER >>>

***
Disclosure: This article was created by the touchDERMATOLOGY team utilizing AI as an editorial tool (ChatGPT (GPT-4o) [Large language model]. https://chat.openai.com/chat.) The content was developed and edited by human editors. No funding was received in the publication of this article.
Editor: Gina Furnival, Senior Editorial Director
Support: No funding was received in the publication of this short article.
Cite: ET-02 shows promising 6-fold hair growth in androgenic alopecia. touchDERMATOLOGY. January 30, 2025.



